
How Can We Use Carbon Dioxide to Feed Rechargeable Batteries?
What if we could capture CO2 and use it to charge the rechargeable batteries? Scientists and engineers have been trying to develop rechargeable batteries using CO2.
Carbon dioxide is one of the major culprits behind climate change. Therefore, the use of renewable energies has continued to increase, and carbon capture technologies have been actively developed, with some being commercialized.
To solve the intermittency problems of renewables, various types of rechargeable batteries have been developed, as well, but none of them have proven sufficient to meeting the storage needs of renewables. In terms of carbon capture technologies, finding clean, efficient ways to utilize captured CO2 has remained a challenge as well.
What if we could capture CO2 and use it to charge the batteries? Capturing CO2 and converting it into electricity, rather than into fuels to burn again. Does that sound like a game changer? Well, we might be able to see such batteries soon; rechargeable batteries using CO2 are being actively studied by scientists and engineers.
To understand how a CO2 battery would work, we should first look at how typical rechargeable batteries operate. Generally speaking, batteries (or cells) are charged and discharged by the conversion of electrical energy into chemical energy and vice versa. When charging, electricity is used to drive chemical reactions (called “redox reactions”) at the electrodes of the battery.
Here, electrical energy is converted into chemical energy. When discharging, the spontaneous redox reaction, which is the reverse of what was driven during the charging process, occurs. This reaction converts chemical energy into electrical energy.
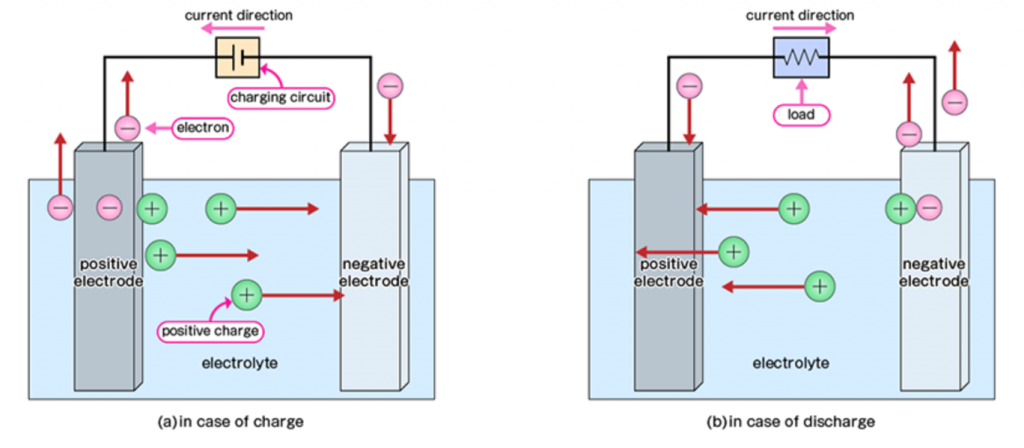
There are some requirements for a molecule to be appropriate for rechargeable batteries, but some typical examples include lead acid, lithium species, nickel/cadmium, and even air.
Yes, air. Quite a number of gases are susceptible to electrochemical reactions, and in fact, metal/air batteries often have higher energy densities than metal, ion, or organic batteries. Although O2 (oxygen gas) and/or H2 (hydrogen gas) are often used for many metal/air batteries such as sodium-oxygen, zinc-oxygen, lithium-oxygen, and nickel-hydrogen, many studies are being done on CO2 batteries as well.
There are a wide variety of CO2 batteries that have been invented, but Li-CO2 battery is the most-studied prototype. In 2013, Lyden A. Archer and his research team at Cornell University introduced the first Li-CO2 battery in their paper “Li-CO2 battery: a novel method for CO2 capture and utilization,” as a method for “simultaneous capturing of CO2 emissions and producing electrical energy.” Although this first Li-CO2 battery wasn’t rechargeable due to the irreversibility of the chemical reactions, this notion of converting CO2 directly into electricity had drawn interests of many scientists.
Accordingly, CO2 electrochemistry, electrode materials, electroactive species, and catalysts for Li-CO2 batteries have been actively studied, and those with higher rechargeability of the battery have been developed, using a doped graphene cathode or Mo2C/CNT catalyst. In 2019, Salehi-Khojin and his research team at the University of Illinois at Chicago reported a fully rechargeable Li-CO2 battery which was stably operated over 500 cycles.
Studies have been ongoing to make it even better; so far, low-cost metal-free catalysts, covalent-organic-framework based Li-CO2 that showed enhanced energy densities, and so on have been reported. Theoretically, Li-CO2 battery can have energy density of 1876 Wh/kg, while that of Li-ion battery is in the range of 200-300 Wh/kg. Although it would take some time and research to approach the theoretical maximum energy density, this potential seems like reason enough to continue researching and developing better Li-CO2 batteries.
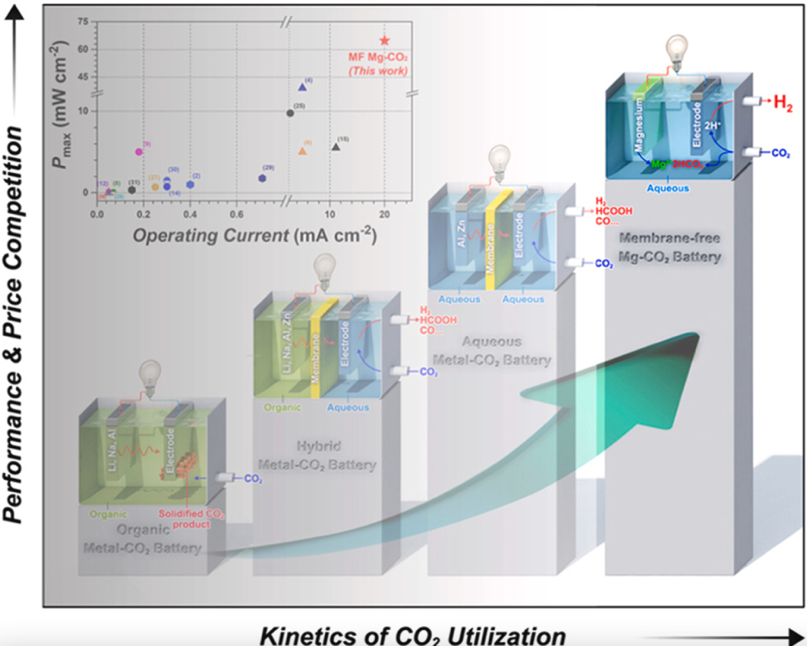
Other than Li-CO2 batteries, some more CO2 rechargeable batteries have been reported. A potassium-CO2 battery reported in 2019 was found to operate stably over 250 cycles, and a sodium-CO2 (Na-CO2) battery reported in 2020 was found to be highly reversible (thus rechargeable) over more than 300 cycles. In 2021, a slightly different type of CO2 battery was reported as well: membrane-free magnesium-CO2 battery used captured CO2 during discharge without generating any wasteful byproducts.
Although there are still some challenges to overcome, the idea of converting CO2 into electricity is exciting. Connecting renewable energies, carbon capture, and energy storage system together can accelerate our journey towards climate mitigation.
This insight is a part of our Undergraduate Seminar Fellows’ Student Blog Series. Read work from other students and learn more about the Undergraduate Climate and Energy Seminar.
Yein Yoon
Undergraduate Seminar FellowYein Yoon is an undergraduate student in the Vagelos Integrated Program in Energy Research (VIPER) studying materials science and engineering and chemistry. Yoon is also a 2021 Undergraduate Seminar Fellow.

