
Ammonia, the Up-and-Coming Carbon-Neutral Fuel
Renewable energy alone cannot provide continuous power. Energy storage is needed to ensure this energy does not go to waste and meet demand. What if ammonia—a common household cleaner— is the answer?
This insight was second place in our fall blog competition.
Reducing carbon dioxide emissions to near zero as quickly as possible is going to take innovative, efficient, and economic solutions, especially considering how intertwined fossil fuel energy is within our daily lives.
Renewable energy, such as solar and wind, have become popular solutions to decarbonizing the energy sector. However, these sources are only available under certain conditions: the sun shining or the wind blowing. What happens when we need electricity on a still night? The answer is energy storage.
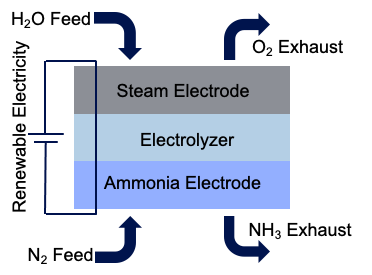
The fuel of the future could be sitting in your own home and has already saved the human race once before: ammonia. Excess renewable electricity can be used to split water into its component parts. Three of the hydrogen atoms that are separated from the water can be bonded with a nitrogen atom to produce ammonia. An electrochemical system that can achieve this is shown in Figure 1.
Today, ammonia is one of the most produced chemicals in the world—nearly USD $60 billion worth—and is mostly used in agricultural fertilizers. Ammonia is primarily produced through the Haber-Bosch process, which relies on natural gas (for the hydrogen) and air (for the nitrogen), making it carbon-intensive, and resulting in CO2 emissions.
It may seem counterintuitive to add another step to the production of hydrogen, since hydrogen is already a carbon-free fuel that could contribute to the energy transition. However, ammonia has many advantages over hydrogen:
- Larger and easier to seal. Ammonia is a larger molecule than hydrogen and therefore is easier to seal in storage containers.
- More space efficient. Ammonia has a higher energy density by volume—nearly twice that of hydrogen—making for more space-efficient storage.
- Easier to transport. Ammonia can be stored as a liquid at room temperature, whereas hydrogen requires expensive cryogenic storage, also complicating its transport.
- Existing knowledge and infrastructure. Due to the industrial scale of existing ammonia production, knowledge of its handling and infrastructure for transport are already widely in place. For the world to switch to a hydrogen economy, additional infrastructure will need to be developed and built worldwide.
- Promise of longer storage capacity. Ammonia production using electrolysis shows promise to store a higher percentage of the generated electricity than gravity storage, and on a longer timeframe than lithium-ion batteries. The longer storage timeframes can be leveraged for seasonal energy storage, to ensure electricity is accessible year-round.
Today, electrolysis is not a process that is often used. This method of hydrogen production is not cost competitive with the traditional route, steam reforming. The main costs for an electrolysis system stem from the capital costs of the electrolyzer and the electricity needed for the separation. Through the use of excess renewable electricity, the costs of electricity can be substantially reduced. Electrolysis powered by low-carbon energy sources does not result in carbon dioxide emissions, unlike steam reforming. If a carbon tax was to be instated, hydrogen from electrolysis would likely become more cost competitive.
In the interim, energy policy is needed to further incentivize electrolysis as a means of renewable energy storage. Currently federal tax credits are awarded for installments of batteries that store solar energy and should be extended to energy storage through electrolysis to encourage “learning by doing.”
In the pursuit of reducing carbon dioxide emissions as quickly as possible, it is evident that energy storage will be needed. Storing excess renewable electricity as ammonia is a strategy to reduce the cost of electrolysis and leverage existing infrastructure for transport—contributing to economic savings and increased viability for adoption. Who would’ve thought a common household cleaning agent could revolutionize the way we harness and distribute electricity?
Maxwell Pisciotta
Student Advisory Council MemberMax Pisciotta is a Ph.D. candidate in the Clean Energy Conversions Lab at Penn’s department for chemical and biomolecular engineering. Their research focuses on carbon capture and carbon removal systems and they were the 2021 Kleinman Birol Fellow.

